Short Report: Onbody Delivery Devices
A continuous evolution in chronic treatment
By: Cindy H. Dubin, Senior Editor, PharmaCircle, Published April 30, 2024

When Rod Stewart wrote “You Wear It Well” in 1972, he most certainly was not singing about onbody drug delivery systems (ODBS). Fifty years later, growing numbers of patients are indeed wearing them well.
The OBDS sector is the offshoot of subcutaneous insulin and diabetes management. First, it was vial and syringe, then prefilled syringes, then autoinjectors as well as insulin pumps along the way. OBS were a further development off of “wearable” insulin pumps offering less complex delivery system and also being smaller and disposable. The first OBS for non-diabetes
indications was Amgens’s Neulasta OnPRO approved in 2014 utilizing Insulet’s OmniPod device originally developed to deliver insulin. This complimented the existing portfolio of PFS and Autoinjector presentations which is a theme in lifecycle management of high value biologics.

Figure 1: Neulasta onPRO OBS (Amgen Neulasta website)
Today, the wearable injector is part of the mainstream drug delivery conversation. This evolution from intravenous to subcutaneous drug delivery could double the value of devices in the latter from approximately $25 billion in 2023 to almost $50 billion by 2033. Patient-centric delivery. Fewer injections. Reduced errors. Increased compliance. Large-volume drug delivery. Management of chronic diseases. Reusable and sustainable. These are among the myriad reasons why both the biopharma industry and the patients it serves are turning to subcutaneous OBDS.
One could say that Skyrizi is a case study of this evolution incorporating both the lifecycle management approach as well as an IV to SC switch for an additional indication. In 2019, Abbvie’s Skyrizi (interleukin-23) was approved in a prefilled syringe for plaque psoriasis. In 2021, an autoinjector version was approved for the same indication. Then, in 2022, Skyrizi received FDA approval as the first and only specific interleukin-23 to treat moderate to severe active Crohn’s in adults. In this case, a 600mg/10mL single-dose vial for IV infusion is offered during the induction period. For maintenance treatment, a 360mg/2.4mL single-dose prefilled cartridge is used with West’s SmartDose onbody injector for subcutaneous injection.

Figure 2: Skyrizi SmartDose OBS (Abbvie Skyrizi website)
Skyrizi represents just one example of the industry’s movement toward more patient-friendly dosing management. Earlier this month, Ypsomed and ten23 health announced they will commercialize the YpsoDose wearable injector for large-volume, subcutaneous delivery. Schott Pharma will provide the cartriQ glass cartridge as the primary packaging.
This past January, Coherus BioSciences, Inc. announced FDA approval of its on-body injector version of Udenyca (pegfilgrastim-cbqv) – a biosimilar to Neulasta Onpro, which first hit the market a decade ago. The five-minute injection with retractable needle will enable patients to receive pegfilgrastim one day after chemotherapy to decrease incidence of infection. The company boasted high customer demand — coupled with confirmed payer coverage — of 138 accounts ordering Udenyca onbody within the first four weeks of launch. An autoinjector version of Udenyca was approved in March 2023.
The Udenyca on-body injector utilizes the LTS Sorrel wearable drug delivery platform. Coherus and LTS Lohmann Therapie-Systeme AG had been working in partnering to receive this approval, whose announcement came just less than six months after LTS acquired Sorrel’s wearable injection device business. Sorrel’s large-volume wearable injector technologies broadens LTS’ portfolio beyond its more passive transdermal and microarray path technologies.
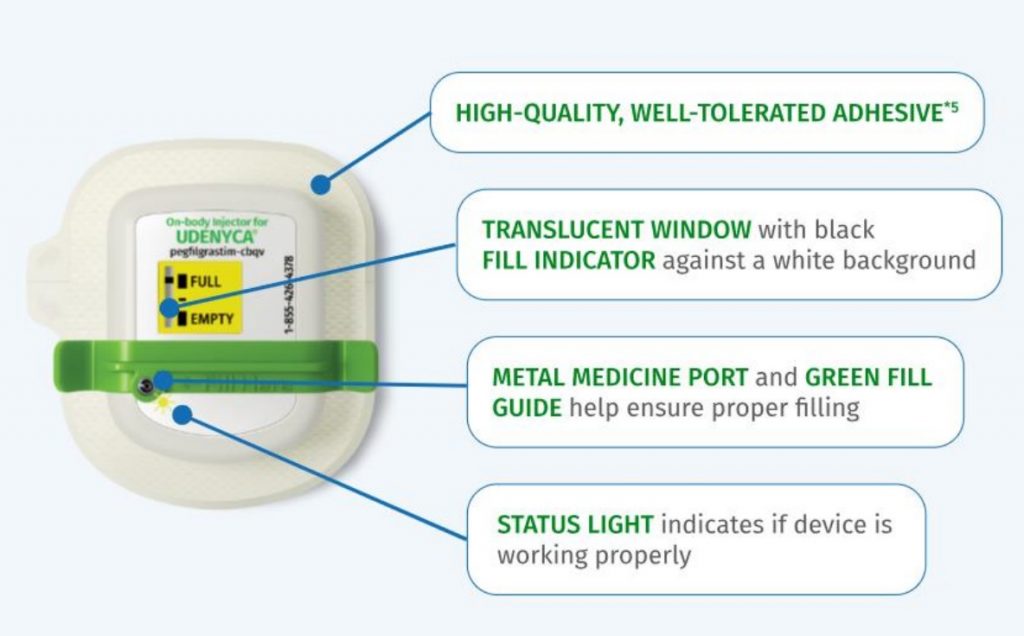
Figure 3: Udenyca OBS (Coherus Undencya website)
In October 2023, Enable Injections received its first stamp of approval of the enFuse injector for the delivery of Empaveli, commercialized by Apellis Pharmaceuticals, to treat adults with paroxysmal nocturnal hemoglobinuria. The Empaveli Injector will streamline self-administration of 20mL of twice-weekly injections each lasting 30 to 60 minutes. Apellis reports a 97% increase in patient compliance.
Enable describes enFuse as a wearable SC injector that combines the ease of use an autoinjector with the volume capacity of a pump. Taking 12 years to develop, enFuse’s FDA approval not only marks the first purely mechanical, large-volume, wearable, subcutaneous drug delivery device, but also expands Enable’s pipeline beyond its traditional focus on thyroid eye disease. TED). Similarly, Enable’s partnership with Viridian Therapeutics, Inc., a biotech focused on rare diseases, will again take the enFuse wearable beyond TED into the automimmune and rare disease space.
enFuse is also being studied as a SC option for isatuximab (Sarclisa), an anti-CD38 monoclonal antibody for patients with relapsed/refractory multiple (RRMM) and for use with FcRn (neonatal crystallizable fragment receptor) inhibitors to treat immunoglobulin G (IgG)-mediated autoimmune disorders (AJMC).
West has been at the forefront of wearable technology for more than a decade, initially commercializing the SmartDose 3.5mL onbody injector and building off of that with the SmartDose 10mL. West’s SmartDose 3.5 is currently used with Amgen’s Pushtronex device to provide single, monthly doses of Amgen’s Repatha. scPharmaceuticals Inc. is incorporating
SmartDose 10 into its Furoscix onbody infuser to treat patients with congestive heart failure. Furoscix is formulated for SC delivery and the SmartDose provides an outpatient alternative. Alexion adopted SmartDose 3.5 to deliver Ultomiris for treating two blood disorders. The Ultomiris Onbody represents a switch from IV to SC delivery as a maintenance treatment using
SmartDose. Two injectors and two injector cartridges provide once weekly dosing.
West Pharmaceutical Services continues to be at the forefront of on-body drug delivery. Earlier this year, patent applications were submitted for a device that researchers claim can painlessly deliver medication through the skin to treat a range of chronic conditions. A prototype has been developed by the Irish arm of West Pharmaceuticals with researchers from Tyndall National Institute and PA Consulting. The researchers explain that the prototype ensures correct depth of medication delivery and dose control using sensors and microneedle technology. Self management of a patient’s drug regimen is connected to the user’s smartphone. A spokesperson at PA Consulting says this is the future of wearable drug delivery systems (Irish Times). The future also includes even more integration with smart technology. The Symbioze smart wearable from Nemera delivers up to 20mL and more of volume, and also has connectivity and live feedback features embedded to ensure proper drug administration.
Making its debut on the trade show circuit last year is Vertiva, Stevanto Group’s next-generation, connected, integrated on-body delivery system that has the ability to switch between basal and bolus injections. The two-part device features a single-use pod that contained a prefilled and preloaded drug cartridge, and a smart controller contains electromechanical elements that can be reused for multiple therapy cycles. Still in the pre-clinical stage, Vertiva has user-interface connectivity and is suitable for a range of SC therapeutic areas and is applicable for complex regimens. In March, Stevanato Group – best known as a pharma packaging company – announced plans to bring the device to market through a collaboration with Thermo Fisher Scientific. The companies plan to offer the platform as an integrated device and fill/finish solution.
Connectivity has become commonplace and device developers will continue to feel the pressure of embedding an element of connectivity as OBDS technology evolves – and that may be something to sing about.
© 2024 PharmaCircle. All rights reserved.
About PharmaCircle

PharmaCircle is a thought leader in drug delivery and formulation development, publishing the Drug Delivery & Formulation Newsletter, which has a worldwide readership, and Drug Delivery Technology Reviews, in-depth reports in all areas of drug delivery including new and novel technologies and devices.
PharmaCircle is also a leading information provider to the pharmaceutical and device industries, providing business prospecting and analysis tools, pipeline and products intelligence, regulatory data, and other information solutions to clients.
PharmaCircle’s Premium Subscripton Service delivers a comprehensive view into drug, biologics and combination product development and innovation, combining scientific, clinical, safety, regulatory, supply chain, and commercial data and insights into one solution.
PharmaCircle’s MedTech Explorer is a comprehensive, hand curated database that assembles the latest intelligence on more than 167,000 medical devices, 112,000 diagnostic tests and 3,300 delivery device technologies in development and on the market from 5,500 companies.
For more information about PharmaCircle and how we can help, contact [email protected] or visit our website at www.pharmacircle.com